
Inovio Clinical Trials Private Limited
Provides medical relief and operates hospitals, nursing homes, and research centers.

Provides medical relief and operates hospitals, nursing homes, and research centers.
Inovio Clinical Trials Private Limited (ICTPL) is a Private Limited Indian Non-Government Company incorporated in India on 16 May 2019 (Five years and 11 months 26 days old ). Its registered office is in Udham Singh Nagar, Uttarakhand, India.
The Corporate was formerly known as Techzombie Solutions Private Limited. The Company is engaged in the Healthcare Industry.
The Company's status is Active, and it has filed its Annual Returns and Financial Statements up until 31 March 2021. It's a company limited by shares with an authorized capital of Rs 0.10 M and a paid-up capital of Rs 0.10 M, as per Ministry of Corporate Affairs (MCA) records.
Soumendra Ray and Sivarajan Chettiar serve as directors at the Company.
Udham Singh Nagar, Uttarakhand, India
+91-XXXXXXXXXX
U74110UR2019PTC009813
009813
Private Limited Indian Non-Government Company
16 May 2019
22 Jun 2021
31 Mar 2021
Unlisted
Roc Uttarakhand
| Name | Designation | Appointment Date | Status |
|---|---|---|---|
| Soumendra Ray | Additional Director | 11-Apr-2022 | Current |
| Sivarajan Chettiar | Director | 25-Nov-2021 | Current |
Inovio Clinical Trials Private Limited, for the financial year ended 2021, experienced significant growth in revenue, with a 36.3% increase. The company also saw a no change in profitability, with a 0% increase in profit. The company's net worth witnessed no change by increase of 0%.
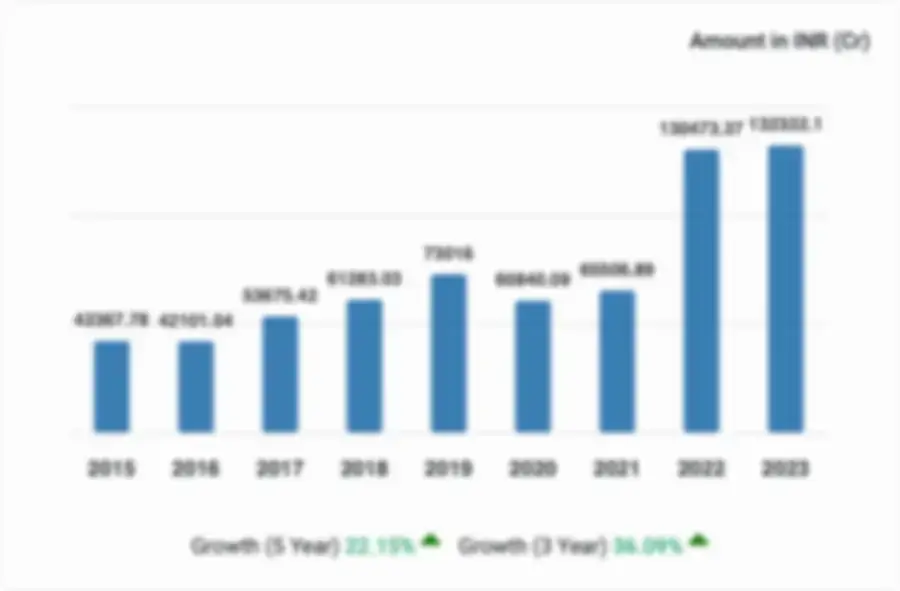
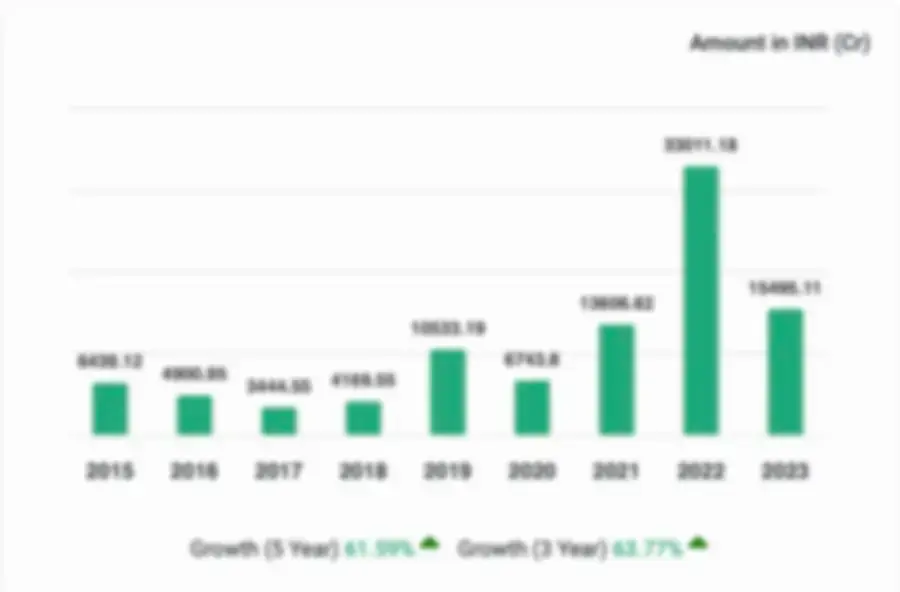
| Metrics |
| (FY 2022) | (FY 2021) | (FY 2020) | (FY 2019) | ||
|---|---|---|---|---|---|---|---|
| Total Revenue |
| ||||||
| Revenue from Operations |
| ||||||
| Total Assets |
| ||||||
| Profit or Loss | |||||||
| Net Worth | |||||||
| EBITDA |
In 2021, Inovio Clinical Trials had a promoter holding of 100.00%. Access key insights, ownership, including shareholding patterns, funding, foreign investors, KMP remuneration, group structure, and overseas investments.

Soumendra Ray is a mutual person

Soumendra Ray is a mutual person

Soumendra Ray and Sivarajan Chettiar are mutual person

Sivarajan Chettiar is a mutual person

There are no open charges registered against the company as per our records.
Unlock and access historical data on people associated with Inovio Clinical Trials, such as employment history, contributions to the Employees' Provident Fund Organization (EPFO), and related information.
Gain comprehensive insights into the Deals and Valuation data of Inovio Clinical Trials, offering detailed information on various transactions, including security allotment data. Explore the intricate details of financial agreements, mergers, acquisitions, divestitures, and strategic partnerships that have shaped Inovio Clinical Trials's trajectory.

Access the credit rating data, providing valuable insights into the company's creditworthiness and financial stability. Explore assessments from leading credit rating agencies, evaluating factors such as debt obligations, liquidity, profitability, and overall financial health.

Stay informed about regulatory alerts and litigation involving and associated companies. Receive timely updates on legal proceedings, regulatory changes, and compliance issues that may impact the company's operations, reputation, and financial performance. Monitor litigation involving subsidiaries, joint ventures, and other affiliated entities to assess potential risks and liabilities.
Soumendra Nath Ray was appointed as a Additional Director was appointed as a Additional Director on 11 Apr 2022 & has been associated with this company since 3 years 30 days .
Sivarajan Thiruganasambandam Chettiar was appointed as a Director was appointed as a Director on 25 Nov 2021 & has been associated with this company since 3 years 5 months .
Inovio Clinical Trials Private Limited last Annual general meeting of members was held on 22 Jun 2021 as per latest MCA records.
Inovio Clinical Trials Private Limited has filed its annual Financial statements for the year ended 31 Mar 2021 with Roc Uttarakhand.
Inovio Clinical Trials Private Limited was registered on 16 May 2019 with Roc Uttarakhand & aged 5 years 11 months as per MCA records.
Inovio Clinical Trials Private Limited was incorporated on 16 May 2019.
The authorized share capital of Inovio Clinical Trials Private Limited is ₹ 0.10 M and paid-up capital is ₹ 0.10 M.
Currently 2 directors are associated with Inovio Clinical Trials Private Limited.
As per Ministry of Corporate Affairs (Mca), the registered address of Inovio Clinical Trials Private Limited is C/O Ramo Devi Khasra No 154 Gadhwal Sabha Jaspur Khurd Road India, Kashipur, Uttarakhand, 244713.
The corporate identification number (CIN) of Inovio Clinical Trials Private Limited is U74110UR2019PTC009813 and the company number is 009813 as per Ministry of Corporate Affairs (MCA).
According to the financial reports for the fiscal year 2021, the revenue trend for Inovio Clinical Trials Private Limited has risen by 36.30%.
The most recent Balance Sheet for Inovio Clinical Trials Private Limited was filed with the ROC on 31 Mar 2021.
Search company & director profiles for free and gain access to critical business data.

RG
MS


_PRIVATE_LIMITED_354462.webp)




