Zenrise Clinical Research Profile
Key Indicators
- Authorised Capital ₹ 7.57 Cr
- Paid Up Capital ₹ 5.43 Cr
- Company Age 7 Year, 7 Months
- Last Filing with ROC 31 Mar 2024
- Open Charges ₹ 19.40 Cr
- Satisfied Charges ₹ 1.40 Cr
- Revenue Growth 16.03%
- Profit Growth -8.74%
- Ebitda -8.35%
- Net Worth 47.57%
- Total Assets 33.91%
About Zenrise Clinical Research
Zenrise Clinical Research Private Limited (ZCRPL) is a Private Limited Indian Non-Government Company incorporated in India on 20 July 2017 (Seven years and seven months 5 days old ). Its registered office is in Hyderabad, Telangana, India.
The Company is engaged in the Pharma Industry.
The Company's status is Active, and it has filed its Annual Returns and Financial Statements up until 31 March 2024. It's a company limited by shares with an authorized capital of Rs 7.57 Cr and a paid-up capital of Rs 5.43 Cr.
The company currently has active open charges totaling ₹19.40 Cr. The company has closed loans amounting to ₹1.40 Cr, as per Ministry of Corporate Affairs (MCA) records.
The Key Managerial Personnel (KMP) at Zenrise Clinical Research Private Limited India is Akula Bapuji as CEO. Srinivas Ravuri, Akula Prasanna, Akula Bapuji, and One other member serve as directors at the Company.
Company Details
- Location
Hyderabad, Telangana, India
- Telephone
+91-XXXXXXXXXX
- Email Address
- Website
- Social Media
Corporate Identity Details
- CIN/LLPIN
U73100TG2017PTC118411
- Company No.
118411
- Company Classification
Private Limited Indian Non-Government Company
- Incorporation Date
20 Jul 2017
- Date of AGM
27 Sep 2024
- Date of Balance Sheet
31 Mar 2024
- Listing Status
Unlisted
- ROC Code
Roc Hyderabad
Industry
What products or services does Zenrise Clinical Research Private Limited offer?
Zenrise Clinical Research Private Limited offers a wide range of products and services, including Audit Assurance Services, Pharma & Bioanalytical Services, Pharmaceutical Services, Chemical Testing Services, Analytical Testing Service, Graduation & High Education Programs, Medical Educational Service, GST & PAN Registrations, GST Consultant, Content Writing Service.
Who are the key members and board of directors at Zenrise Clinical Research?
Executive Team (1)
| Name | Designation | Appointment Date | Status |
|---|---|---|---|
| Akula Bapuji | CEO | 19-Dec-2018 | Current |
Board Members (4)
| Name | Designation | Appointment Date | Status |
|---|---|---|---|
| Srinivas Ravuri | Director | 20-Jul-2017 | Current |
| Akula Prasanna | Whole-Time Director | 19-Mar-2018 | Current |
| Akula Bapuji | Whole-Time Director and Ceo | 24-Jul-2018 | Current |
| Loganathan Sekar | Director | 29-Jan-2024 | Current |
Financial Performance of Zenrise Clinical Research.
Zenrise Clinical Research Private Limited, for the financial year ended 2023, experienced significant growth in revenue, with a 16.03% increase. The company also saw a slight decrease in profitability, with a 8.74% decrease in profit. The company's net worth Soared by an impressive increase of 47.57%.
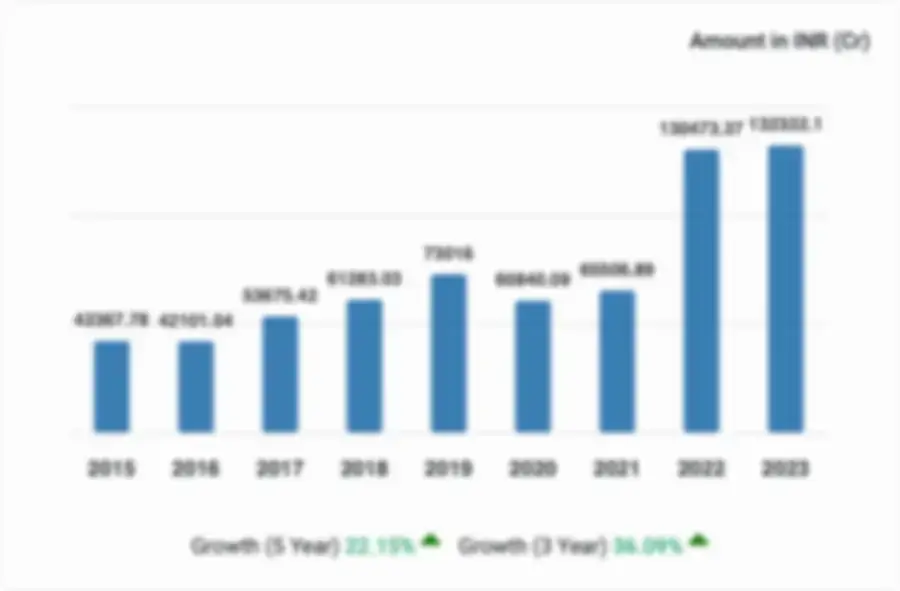
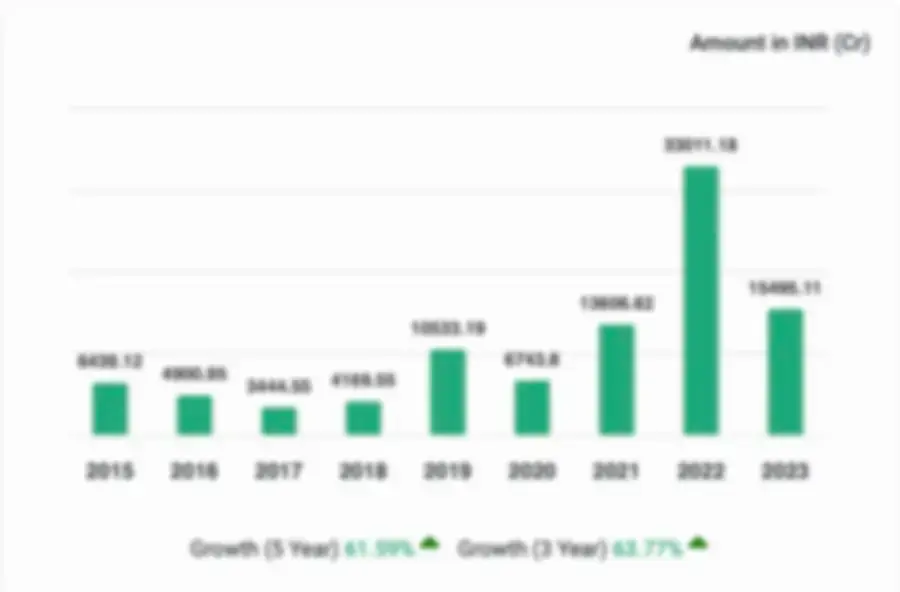
- Key Matrics
- Balance Sheet
- Profit and Loss
- Cash Flow
- Ratios
| Metrics |
| (FY 2022) | (FY 2021) | (FY 2020) | (FY 2019) | ||
|---|---|---|---|---|---|---|---|
| Total Revenue |
| ||||||
| Revenue from Operations |
| ||||||
| Total Assets |
| ||||||
| Profit or Loss |
| ||||||
| Net Worth |
| ||||||
| EBITDA |
|
What is the Ownership and Shareholding Structure of Zenrise Clinical Research?
In 2023, Zenrise Clinical Research had a promoter holding of 85.93% and a public holding of 14.07%. Access key insights, ownership, including shareholding patterns, funding, foreign investors, KMP remuneration, group structure, and overseas investments.
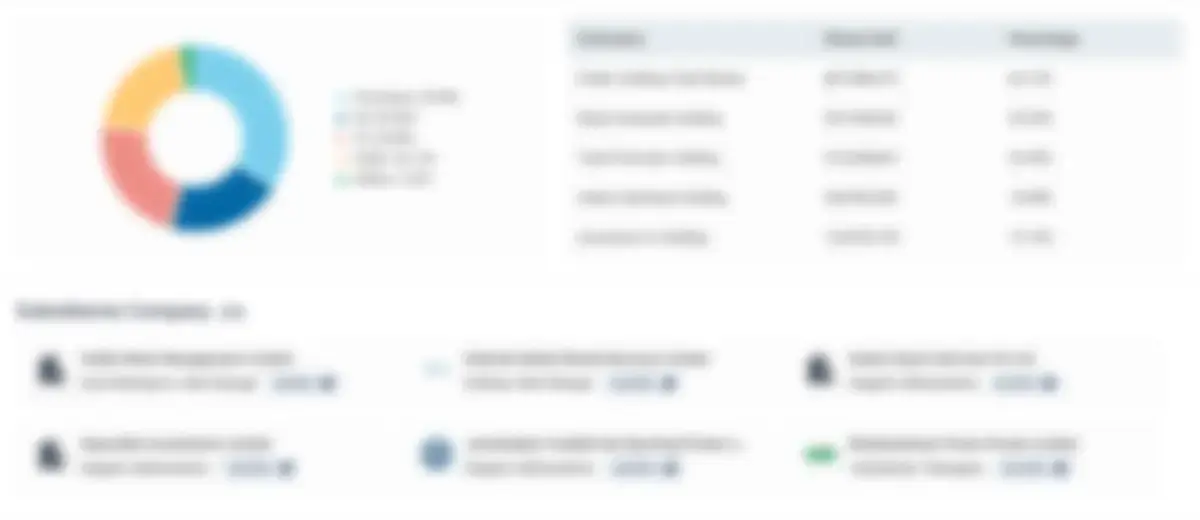
Charges (Loans)
₹19.40 Cr
₹1.40 Cr
Charges Breakdown by Lending Institutions
- Others : 19.40 Cr
Latest Charge Details
| Date | Lender | Amount | Status |
|---|---|---|---|
| 21 Oct 2023 | Others | ₹11.40 Cr | Open |
| 25 Jan 2019 | Others | ₹8.00 Cr | Open |
| 13 Aug 2021 | State Bank Of India | ₹1.40 Cr | Satisfied |
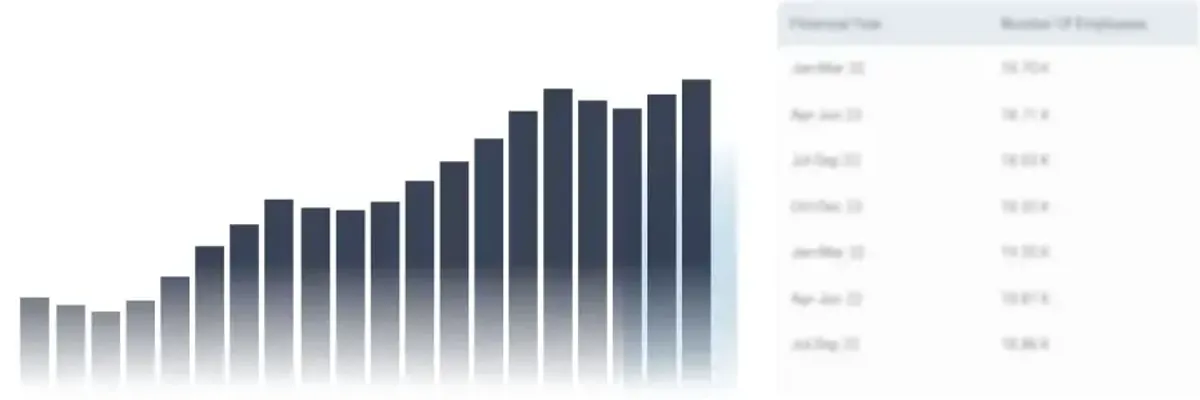
How Many Employees Work at Zenrise Clinical Research?
Zenrise Clinical Research has a workforce of 148 employees as of Mar 22, 2024. Unlock access to detailed historical data on individuals associated with the company, including employment records, contributions to the Employees' Provident Fund Organization (EPFO), and other related insights.
Deals i
Gain comprehensive insights into the Deals and Valuation data of Zenrise Clinical Research, offering detailed information on various transactions, including security allotment data. Explore the intricate details of financial agreements, mergers, acquisitions, divestitures, and strategic partnerships that have shaped Zenrise Clinical Research's trajectory.
Rating

Access the credit rating data, providing valuable insights into the company's creditworthiness and financial stability. Explore assessments from leading credit rating agencies, evaluating factors such as debt obligations, liquidity, profitability, and overall financial health.
Alerts

Stay informed about regulatory alerts and litigation involving and associated companies. Receive timely updates on legal proceedings, regulatory changes, and compliance issues that may impact the company's operations, reputation, and financial performance. Monitor litigation involving subsidiaries, joint ventures, and other affiliated entities to assess potential risks and liabilities.
